When we refer to crude oil as a raw material for the chemical industry, we are usually referring to crude oil, which a mixture of hydrocarbons. Strictly, we should be using the term petroleum, derived from Latin petra – rocks and oleum – oil. Petroleum describes not only the mixture of hydrocarbons in crude oil, including the gases and solids which are dissolved in the liquid but also any free gas, known as natural gas, associated with it.
- This unit describes how petroleum is formed and outlines the drilling techniques which are used to extract it.
- In another unit, the method of separating petroleum into separate fractions in a refinery by distillation is outlined.
- A third unit is devoted to the other processes used in in a refinery: cracking, isomerisation, reforming and alkylation. These processes produce gaseous and liquid fuels and the compounds needed in the chemical industry to make a vast number of products from plastics to medicines.
Petroleum that is worthwhile extracting is usually found trapped in layers of permeable rocks by other layers of impermeable rock, but more recently reserves of gas and oil are being extracted from shale which is an impermeable rock but is porous in the sense that there are spaces (pores) within its structure in which liquids and gases can be trapped.
Formation of natural gas and crude oil
Well over 200 different hydrocarbons can be identified in a sample of crude oil. They were formed in remote periods of geological time, anything from 50 to 500 million years ago, from the remains of living organisms. It is, therefore, a fossil fuel.
Weathered rock material, eroded from land masses and carried to the sea, accumulated in layers over millions of years in subsiding basins, and the remains of large quantities of marine plant and animal organisms became incorporated in the sediment (Figure 1).
Owing to the thickness of the sediments, high pressures built up which, probably in conjunction with biochemical activity, led to the formation of petroleum. The detailed mechanism is obscure, but it is probable that anaerobic microbes lowered the oxygen and nitrogen content of what had been living matter.
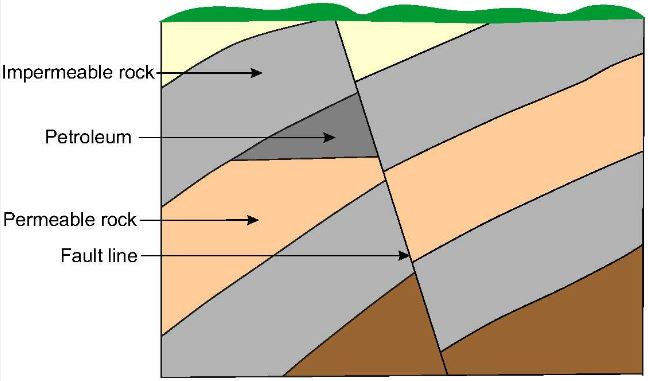
Subsequent earth movements which caused uplift of the sedimentary basins also caused migration of the petroleum through pores in the rocks, sometimes to areas far from where it was formed. In the course of migration, some of the petroleum accumulated in traps where the permeable rock was bounded by impermeable rock. The principal types of trap in oil fields found all over the world are the anticline (an upfold in the strata) as shown in Figure 1, the fault trap (Figure 2) and the salt dome (Figure 3).
 |
 |
|
| Figure 1 An anticline is where previously flat strata have been bent upwards by earth movements to form an arch. In this case the petroleum has migrated upwards in the permeable rock and become trapped by the overlying impermeable rock. | Figure 2 A fault line is the line along which the strata on one side has been displaced and is no longer aligned with the strata on the other side. In the example depicted here a layer of impermeable rock has trapped the petroleum by preventing it migrate further in the layer of permeable rock./span |
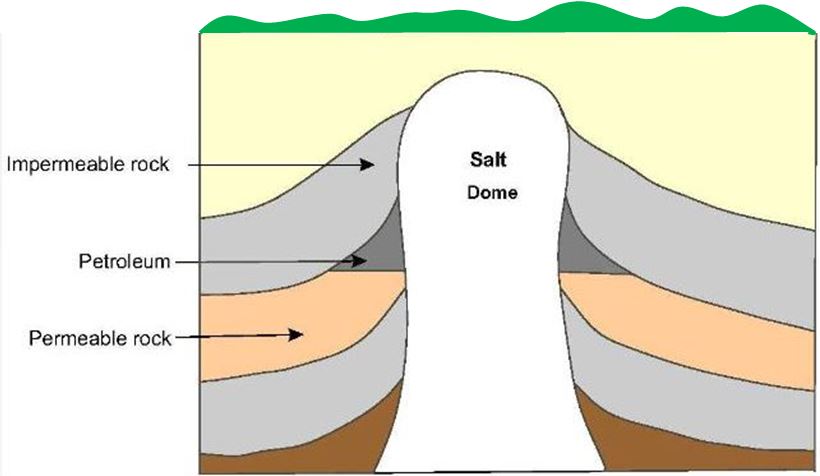 |
| Figure 3 Rock salt, when subjected to heat and pressure, can move very slowly upwards forcing its way through the overlying rock strata and so forming a salt dome. In the case shown, petroleum in the layer of permeable rock has become trapped by the overlying impermeable rock and the salt dome. |
Because the liquid oil and associated gas are trapped, in large amounts, into one area of permeable rock, it is possible to drill vertically into this rock and the oil and gas, under pressure rises up a pipe to the surface. The gas is separated from the oil and the crude oil is then said to be stabilized. The gas and oil are then transported by pipes either by land to a refinery or to a ship (tanker). If they are being transported by ship, the gas is liquefied before being pumped into the tanker. So that the tankers can readily offload the gas and oil, refineries all over the world are built near the coastline.
The liquid oil contains mainly alkanes (with 5 to about 125 carbon atoms in the molecules), cycloalkanes and aromatic hydrocarbons. The relative amounts of the three classes of compound vary with the oil-field, alkanes (15% – 60%), cycloalkanes (30% – 60%), aromatics (3% to 30%), with a residue of very high molecular mass hydrocarbons (e g bitumen) making up the remainder.
The average length of the carbon chains also varies from field to field. In some areas, there is preponderance of smaller hydrocarbon molecules (light crude oil). In heavy crude oil, there is a greater proportion of larger molecules.
Natural gas is principally methane, with smaller amounts of other alkanes, ethane, propane and the butanes. As with liquid oil, the composition of natural gas varies from field to field. In some fields, methane may make up 98% of the gas and it is known as dry natural gas. In wet natural gas, as much as 20% of the gas is made up of other alkanes, ethane, propane and the butanes. Some natural gas, as in Southern France, contains large amounts, up to 16%, of hydrogen sulphide, and others, as in the US, considerable amounts of helium. In some fields, the natural gas contains up to 7% of helium by volume.
Many of the oil fields are located off-shore which presents additional challenges.


